This artifact shows excerpts from lesson plans showing three different ways I explained how polymers are formed in Scientific Thinking 1.
This artifact supports the indicator Differentiated Instruction and Assessment (4) provides alternative explanations of course concepts.
Method 1: Building Class Molecular Model
“Now that we’ve tested some plastics and know them intimately, we’re going to zoom in and talk a little bit about the molecular structure. There was a preview of this in the Plastics 101 video…
Activity 19 in book; Today, do the first part (p. B-89 in teacher’s binder)
Give them: 4 H, 2 C, 1 O and 7 bonds (per student)
Ask them “What type of molecule can you make using ALL of these atoms and bonds? Remind them to connect all bonding sites; allow them to try different combinations.
Eventually they will come up with:
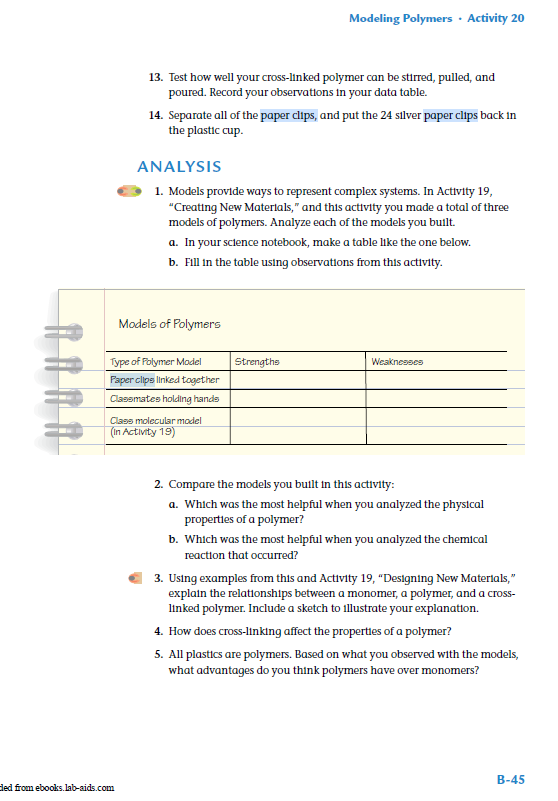
This is vinyl alcohol
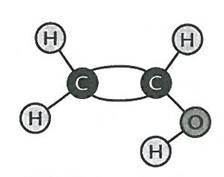
Tell them they can use all of these single molecules to build a long chain. Distribute more white bonding tubes. Have students at table group bond their three molecules together, (to do they will need to break the double bond between the carbon molecules) then bond with table group across from them, and then with whole class
Molecule will look like:
There will be an unbonded spike at each end (from carbon)
You have chemically bonded your one molecule into a long chain of many vinyl alcohols
This models the chemical reaction of vinyl alcohol into polyvinyl alcohol. Called polyvinyl because “poly” means “many”
In a reaction like this the starting subunit is called the monomer and the chain of many parts is the polymer
Show bottle of PVA. Explain that model is actually molecular model of this substance.”
Method 2: Class Holding Hands Model
“More Modeling Polymers
Today we’re going to add in one more concept–that of crosslinking polymers.
So we used plastic balls to model molecules the other day. Now creating another model.
Head outside with students if it’s a nice day and find some things to walk through. Otherwise, do it in the classroom.
If you go outside, make sure to prep students to stay focused and not get too crazy. HUGE rule: no running!
Ask for two volunteers: a pair of students holding both hands represents a double bonded vinyl alcohol monomer. How can we represent the formation of a polymer using this approach? A line of students holding hands can represent a polymer.
Start with student pairs, have them walk through doorway or pair of desks and return. Then make (ideally) 6-8 person polymer chains and repeat action. Tell them that this is the PVA that they used to make the product in the last lab.
Finally, have two or more students hold together two 6-8 person chains to represent a cross-linked polymer. Have this human cross-linked polymer walk through desks or doorway and return. Tell them that this is what happened when they added the sodium borate to the PVA.
What effect did going from monomers to polymers have on your ability to move? Traveling in a monomer pair was much easier than as a polymer. However, the chain can still pass relatively easily through the door way. A polymer chain is still fairly flexible. How did cross-linking affect your rate of travel? The longer and more complex the chain, the slower the progress.
Head back inside to continue work.
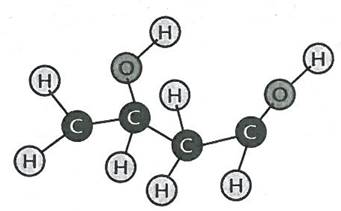
Show this crosslinking diagram on the board and explain the reaction:
Method 3: Paperclip Models (lab from Lab-Aids.com)